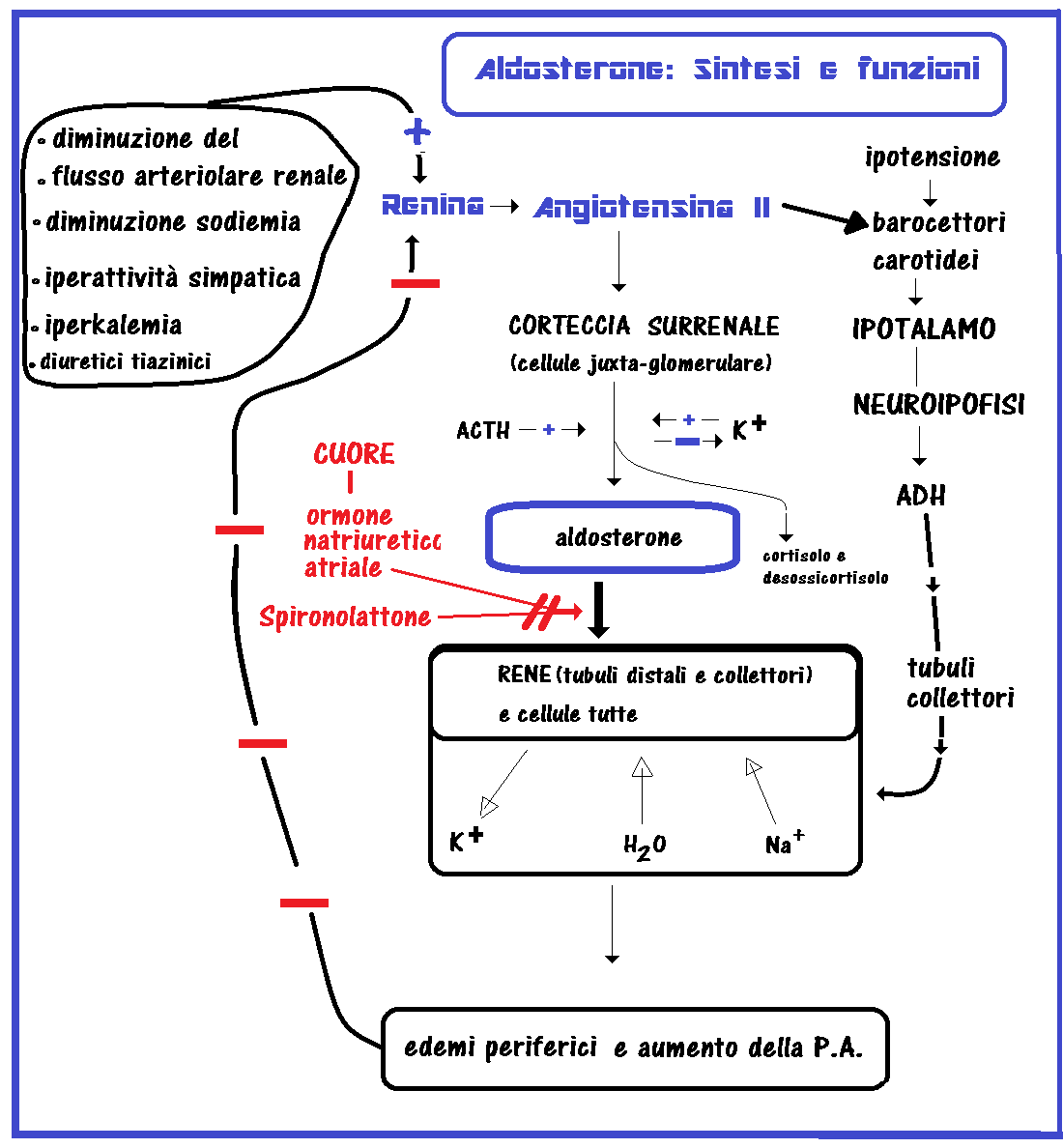
L’aldosterone, identificato nel 1953, è il principale ormone mineralcorticoide presente in circolo, esso è reposto alla regolarizzazione del volume dei liquidi extracellulari e dei livelli sierici di sodio e di potassio. E’ prodotto nella zona juxta-glomerulare della corteccia surrenale con l’ausilio dell’enzima aldosterone-sintetasi (CYP11B2) (1-5).
L’aldosterone rappresenta l’effettore finale del sistema renina-angiotensina-aldosterone (RAA). Il sistema RAA e il potassio sono i maggiori regolatori, mentre l’ormone adrenocorticotropo (ACTH), il sodio, vasopressina, dopamina, peptide natriuretico atriale, sostanze adrenergiche, serotonina e somatostatina sono modulatori minori (6-8).
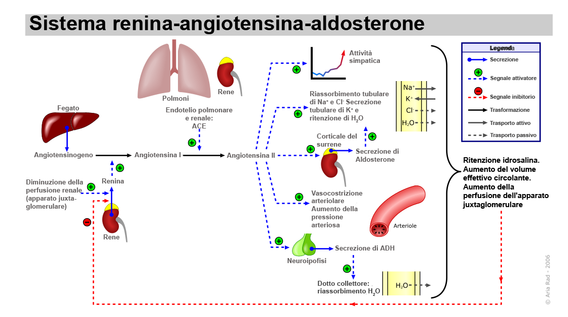
La renina, un enzima prodotto dalle cellule juxtaglomerulari renali, interviene nel clivaggio della macroglobulina epatica angiotensinogeno che si trasforma in angiotensina I. L’angiotensina I a sua volta viene rapidamente trasformata dall’enzima di conversione dell’angiotensina (ACE) , prodotto dai polmoni, nell’octapeptide angiotensina II. Il clivaggio del residuo NH-terminale dell’angiotensina II produce angiotensina III. Mentre angiotensina I non ha attività biologica nota, l’angiotensina II e l’angiotensina III stimolano la secrezione di aldosterone in modo simile, sebbene l’angiotensina II sia un agente vasocostrittore più potente. Le angiotensine vengono poi inattivate nell’arco di alcuni minuti ad opera di peptidasi plasmatiche e tissutali. Le angiotensine provocano anche la secrezione di cortisolo e deossicorticosterone, anche se in misura molto minore (9-22).
La secrezione della renina è stimolata dalla riduzione del volume ematico e del flusso nelle arteriole afferenti renali, dalla riduzione della concentrazione del sodio nel fluido tubulare, percepito dalla macula densa, e dall’iperattività del sistema nervoso simpatico renale. Fattori che riducono il flusso renale sono: disidratazione, emorragie, restrizione sodica, posizione ortostatica o stenosi delle arterie renali e questi fattori aumentano la secrezione di renina (23-34).
Al contrario, fattori che aumentano il flusso renale, come aumentato introito di sodio, vasocostrizione periferica, o posizione clinostatica, riducono la secrezione di renina. Anche l’angiotensina II inibisce la secrezione di renina tramite un meccanismo diretto di feedback negativo. L’ipokaliemia aumenta e l’iperkaliemia riduce il rilascio di renina (35-46).
L’aldosterone riduce i livelli di renina tramite un’azione indiretta attraverso meccanismi di ritenzione idrosalina ed espansione di volume.
Tra i fattori ipofisari va ricordato l’ACTH, che stimola la secrezione di aldosterone in modo acuto ma
non cronico.
FUNZIONI DELL’ALDOSTERONE: regola il bilancio elettrolitico e idrico aumentando la ritenzione renale di sodio e l’escrezione di potassio a livello dei tubuli distali e collettori del rene. Su tutte le cellule dell’organismo agisce facilitando l’ingresso dell’acqua e del sodio e la fuoriuscita del potassio (contribuendo così a riequilibrare riportandoli alla norma, valori pressori bassi) (47-55).
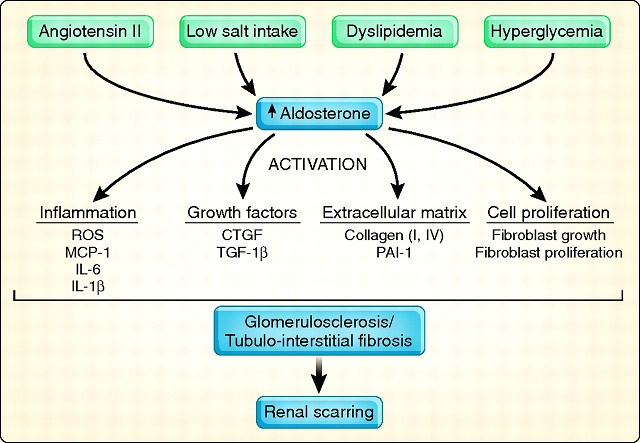
 Inizialmente si ritenne che l’azione esclusiva dell’aldosterone fosse quella sodio-ritentiva a livello renale, in seguito l’attenzione sull’aldosterone fu ampliata per il suo ruolo nello sviluppo di processi infiammatori e fibrotici nell’ambito di svariate patologie cardiovascolari.
Inizialmente si ritenne che l’azione esclusiva dell’aldosterone fosse quella sodio-ritentiva a livello renale, in seguito l’attenzione sull’aldosterone fu ampliata per il suo ruolo nello sviluppo di processi infiammatori e fibrotici nell’ambito di svariate patologie cardiovascolari.
IPERALDOSTERONISMO – patologia di non comune riscontro; interessa prevalentemente i soggetti di sesso femminile di età compresa fra i 30 e i 50 anni. Esistono varie forme di iperaldosteronismo che, essenzialmente, possiamo distinguere in due categorie: primario e secondario (44-60).
Iperaldosteronismo primario: sindrome caratterizzata da ipersecrezione di aldosterone provocata da patologia interna al surrene: iperplasia surrenalica, adenoma o carcinoma. Esistono varie forme di iperaldosteronismo primario; la più comune (60% dei casi circa), tanto da esserne considerata un sinonimo è la sindrome di Conn in cui è presente un  adenoma surrenalico.
adenoma surrenalico.
L’iperaldosteronismo primario idiopatico è presente nel 40% circa dei casi ed è dovuto a iperplasia surrenalica bilaterale. L’iperplasia delle surrenali può presentarsi in due tipi: tipo I (sopprimibile con desametasone) e tipo II.
Piuttosto rari sono i casi di iperaldosteronismo primario dovuti a carcinoma secernente aldosterone e a iperplasia monolaterale.
Iperaldosteronismo secondario: dovuto ad una iperattività del sistema renina-angiotensina, da iperplasia fibromuscolare, stenosi dell’arteria renale, tumore delle cellule iuxta-glomerulari e altre cause summenzionate che inducono iperattivazione del sistema RAA.
Altri fattori che influenzano i valori dell’aldosteronemia sono la postura, lo stress e la gravidanza. Anche le variazioni del sodio nella dieta possono influire sui livelli plasmatici di aldosterone.
Sintomatologia dell’iperaldosteronismo:
- iperaldosteronemia
- ipokalemia, alcalosi ipokaliemica, nefropatia ipokalemica
- ipernatriemia,

- ipervolemia
- astenia,
- cefalea,
- parestesie,
- paralisi transitoria
- tetania.
- ipertensione diastolica; In molti casi, il solo sintomo è un’ipertensione da lieve a moderata.
- poliuria
- polidipsia
- Gonfiore del viso (facies lunaris, cushingoide): viso tondo per l’imbibizione dei tessuti, rosso-cianotico, acne, rima labiale ristretta (a bocca di pesce), capelli sfibrati e grassi.
- edemi periferici, poco frequenti.
Diagnostica strumentale e di laboratorio:
- aldosteronemia: In media, poiché esistono ampie variazioni, 10-100 ng/L nel soggetto sdraiato e 70-300 ng/L nel soggetto in piedi.
- Attività reninica plasmatica elevata
- Elettroliti: Na+, K+
- Diagnostica per immagini del surrene: PET-TAC
- Cateterizzazione bilaterale delle vene surrenaliche (per dosare i livelli di cortisolo e aldosterone): adrenal venous sampling
- Scintigrafia
TERAPIA DELL’IPERALDOSTERONISMO
- rimozione chirurgica dei tumori – Tra i pazienti con surrenectomia bilaterale per iperplasia surrenalica, il 70% dei pazienti continua a essere iperteso; pertanto il trattamento chirurgico non è raccomandato in caso di iperplasia.
- L’iperaldosteronismo in questi pazienti può generalmente essere controllato con un bloccante selettivo dell’aldosterone come lo spironolattone (Spirolang® cpr, Aldactone® cpr 100 mg), iniziando con 50 mg PO 1 volta/die e aumentando poi la dose in 1-3 mesi sino a quella di mantenimento, solitamente intorno ai 100 mg 1 volta/die (61-73). Molto utilizzato l’amiloride, che inibisce il riassorbimento di sodio e l’escrezione di potassio, in associazione con diuretici tiazidici che inibiscono il riassorbimento di sodio e aumentano l’escrezione di acqua (Moduretic® cpr 5 + 50 mg) 5-10 mg PO 1 volta/die (71-75). Un altro diuretico risparmiatore di K è l’eplerenone (Inspra® cpr 25, 50 mg) ad una dose compresa tra i 50 mg PO 1 volta/die e i 200 mg PO bid può essere utilizzato perché, a differenza dello spironolattone, non blocca il recettore degli androgeni; perciò esso è il farmaco di scelta per il trattamento a lungo termine nei maschi (74-122)
Ipoaldosteronismo: La secrezione di aldosterone diminuisce nell’iperplasia surrenalica congenita, nell’insufficienza corticosurrenalica primitiva (morbo di Addison), deficit di aldosterone-sintetasi, (CYP11B2), utilizzo di farmaci (alcuni diuretici, antinfiammatori non steroidei o FANS, ACE inibitori, ciclosporina). nella sindrome di Turner, nell’etilismo acuto e nel diabete mellito. “Ipoaldosteronismo isolato” viene utilizzato per descrivere bassi livelli di aldosterone senza cambiamenti corrispondenti nei livelli di cortisolo e si differenzia dall’Ipoaldosteronismo iporeninemico in cui coesistono diminuzione della produzione di angiotensina 2 e disfunzioni intrasurrenaliche (120-129).
Terapia:
- Il deficit di Aldosterone dovrebbe essere trattato con un mineralocorticoide (come ad esempio il fludrocortisone, Florinef® cpr 0.1 mg), e possibilmente con un glucocorticoide per il deficit di cortisolo, se presente.
- L’ipoaldosteronismo iporeninemico è suscettibile di trattamento con fludrocortisone (Florinef® cpr 0.1 mg), ma la coesistenza di ipertensione ed edema in questi pazienti richiede associazione di terapia con la bendroflumetiazide (Aprinox® cpr), La bendroflumetiazide è un diuretico tiazidico che agisce a livello del tubulo contorto prossimale dove avviene il 65% della filtrazione dell’acqua. I tiazidici inibiscono l’anidrasi carbonica inibendo in tal modo la metabolizzazione del bicarbonato HCO3– e manacata formazione di CO2 e H+. In tal modo Na+, HCO3– e H2O non vengono riassorbiti a livello del tubulo contorto prossimale. Ma verranno comunque in gran parte riassorbiti a livello dell’ansa di Henle, a livello del tubulo contorto distale e del tubulo collettore. La bendroflumetiazide inibisce anche la secrezione di latte di latte materno nelle donne ed è talvolta usato per questo scopo.
Effetti collaterali dei diuretici: astenia, ipotensione, stanchezza cronica, lipotimie, vertigini, poliuria, aumento della sete, acidosi metabolica, calcolosi renale.
Oltre ai diuretici tiazidici si possono utilizzare i diuretici dell’ansa, come la furosemide (Lasix® cpr 25 mg).
References:
- Lv, Y., Bai, S., Zhang, H., Zhang, H., Meng, J., Li, L., Xu, Y. Aldosterone down-regulates the slowly activated delayed rectifier potassium current in adult guinea pig cardiomyocytes. British Journal of Pharmacology 2015; 172(-23-)
- Shi, H., Zhang, A., He, Y., Yang, M., Gan, W. Effects of p53 on aldosterone-induced mesangial cell apoptosis in vivo and in vitro. Mol Med Rep 2016; 13(-6-):5102-8.
- Sheng, L., Yang, M., Ding, W., Zhang, M., Niu, J., Qiao, Z., Gu, Y. Epidermal growth factor receptor signaling mediates aldosterone-induced profibrotic responses in kidney. Exp Cell Res 2016; 346(-1-):99-110.
- Sakamoto, T., Fujii, A., Saito, N., Kondo, H., Ohuchi, A. Alteration of amiloride-sensitive salt taste nerve responses in aldosterone/NaCl-induced hypertensive rats. Neurosci Res 2016; 108(–):60-6.
- Wang, B., Xu, X., He, X., Wang, Z., Yang, M. Berberine Improved Aldo-Induced Podocyte Injury via Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress Pathways both In Vivo and In Vitro. Cell Physiol Biochem 2016; 39(-1-):217-28.
- Andrew S. Terker, Chong Zhang, Kayla J. Erspamer, Gerardo Gamba, Chao-Ling Yang, David H. Ellison Kidney Int 2016; 89(–):127-134.
- Koneru, B., Bathina, C.S., Cherry, B.H., Mifflin, S.W. Mineralocorticoid receptor in the NTS stimulates saline intake during fourth ventricular infusions of aldosterone. AMERICAN JOURNAL OF PHYSIOLOGY-REGULATORY INTEGRATIVE AND COMPARATIVE PHYSIOLOGY 2014; 306(-1-)
- Clayton, S.C., Zhang, Z.M., Beltz, T., Xue, B.J., Johnson, A.K., Johnson, A.K. CNS neuroplasticity and salt-sensitive hypertension induced by prior treatment with subpressor doses of ANG II or aldosterone1523. AMERICAN JOURNAL OF PHYSIOLOGY-REGULATORY INTEGRATIVE AND COMPARATIVE PHYSIOLOGY 2014; 306(–):
- Zhang, Y., Robson, S.C., Morris, K.L., Heiney, K.M., Dwyer, K.M., Kishore, B.K., Ecelbarger, C.M., Kishore, B.K. Impaired natriuretic response to high-NaCl diet plus aldosterone infusion in mice overexpressing human CD39, an ectonucleotidase (NTPDase1). AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY 2015; 308
- Thunhorst, R.L., Xue, B.J., Beltz, T.G., Johnson, A.K., Thunhorst, R.L. Age-related changes in thirst, salt appetite, and arterial blood pressure in response to aldosterone-dexamethasone combination in rats. AMERICAN JOURNAL OF PHYSIOLOGY-REGULATORY INTEGRATIVE AND COMPARATIVE PHYSIOLOGY 2015; 308
- Sawada, H., Naito, Y., Oboshi, M., Iwasaku, T., Okuhara, Y., Morisawa, D., Eguchi, A., Hirotani, S., Masuyama, T., Naito, Y. Iron restriction inhibits renal injury in aldosterone/salt-induced hypertensive mice. HYPERTENSION RESEARCH 2015; 38(–):317-322.
- Martinez-Martinez, E., Cachofeiro, V., Rousseau, E., Alvarez, V., Calvier, L., Fernandez-Celis, A., Leroy, C., Miana, M., Jurado-Lopez, R., Briones, A.M., Jaisser, F., Zannad, F., Rossignol, P., Lopez-Andres, N., Lopez-Andres, N. Interleukin-33/ST2 system attenuates aldosterone-induced adipogenesis and inflammation. MOLECULAR AND CELLULAR ENDOCRINOLOGY 2015; 411(–):20-27.
- Ding, W., Xu, C.Y., Wang, B., Zhang, M.M., Zhang, M.M. Rotenone Attenuates Renal Injury in Aldosterone-Infused Rats by Inhibiting Oxidative Stress, Mitochondrial Dysfunction, and Inflammasome Activation. MEDICAL SCIENCE MONITOR 2015; 21(–):U1-U8.
- Ichikawa, D., Kamijo-Ikemori, A., Sugaya, T., Shibagaki, Y., Yasuda, T., Hoshino, S., Katayama, K., Igarashi-Migitaka, J., Hirata, K., Kimura, K., Kamijo-Ikemori, A. Human liver-type fatty acid-binding protein protects against tubulointerstitial injury in aldosterone-induced renal injury. AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY 2015; 308(–)
- Yang, M., Wang, B., Li, M., Jiang, B., Yang, M. Connexin 43 is Involved in Aldosterone-Induced Podocyte Injury. CELLULAR PHYSIOLOGY AND BIOCHEMISTRY 2014; 34(–):1652-1662.
- Wang, B., Ding, W., Zhang, M.M., Li, H.M., Gu, Y., Gu, Y. Rapamycin Attenuates Aldosterone-Induced Tubulointerstitial Inflammation and Fibrosis. CELLULAR PHYSIOLOGY AND BIOCHEMISTRY 2015; 35(–):116-125. >>>
- Tanaka, K., Wilson, R.M., Essick, E.E., Duffen, J.L., Scherer, P.E., Ouchi, N., Sam, F., Sam, F. Effects of Adiponectin on Calcium-Handling Proteins in Heart Failure With Preserved Ejection Fraction. Circulation-Heart Failure 2014; 7(–):976-U166.
- Queisser, N., Oteiza, P.I., Link, S., Hey, V., Stopper, H., Schupp, N., Queisser, N. Aldosterone Activates Transcription Factor Nrf2 in Kidney Cells Both In Vitro and In Vivo. ANTIOXIDANTS&REDOX SIGNALING 2014; 21(–):2126-2142.
- Queisser, N., Happ, K., Link, S., Jahn, D., Zimnol, A., Geier, A., Schupp, N., Schupp, N. Aldosterone induces fibrosis, oxidative stress and DNA damage in livers of male rats independent of blood pressure changes. TOXICOLOGY AND APPLIED PHARMACOLOGY 2014; 280(–):399-407.
- Gravez, B., Tarjus, A., Pelloux, V., Ouvrard-Pascaud, A., Delcayre, C., Samuel, J., Clement, K., Farman, N., Jaisser, F., Messaoudi, S., Messaoudi, S. Aldosterone Promotes Cardiac Endothelial Cell Proliferation In Vivo. JOURNAL OF THE AMERICAN HEART ASSOCIATION 2015; 4(–):
- Chrissobolis, S., Drummond, G.R., Faraci, F.M., Sobey, C.G., Chrissobolis, S. Chronic aldosterone administration causes Nox2-mediated increases in reactive oxygen species production and endothelial dysfunction in the cerebral circulation. Journal of Hypertension 2014; 32(–):1815-1821.
- Zhang, X.L., Liu, J., Pang, X.M., Zhao, J.J., Wang, S.Y., Wu, D., Liu, J. Aldosterone induces C-reactive protein expression via MR-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. MOLECULAR AND CELLULAR ENDOCRINOLOGY 2014; 395(–):61-68.
- Ohsawa, M., Tamura, K., Wakui, H., Maeda, A., Dejima, T., Kanaoka, T., Azushima, K., Uneda, K., Tsurumi-Ikeya, Y., Kobayashi, R., Matsuda, M., Uchida, S., Toya, Y., Kobori, H., Nishiyama, A., Yamashita, A., Ishikawa, Y., Umemura, S., Tamura, K. Deletion of the angiotensin II type 1 receptor-associated protein enhances renal sodium reabsorption and exacerbates angiotensin II-mediated hypertension. Kidney International 2014; 86(–):570-581.
- Rock, M.L., Karas, A.Z., Rodriguez, K.B.G., Gallo, M.S., Pritchett-Corning, K., Karas, R.H., Aronovitz, M., Gaskill, B.N. The Time-to-Integrate-to-Nest Test as an Indicator of Wellbeing in Laboratory Mice. Journal of the American Association for Laboratory Animal Science 2014; 53(-1-):24-28.
- T., Doi, S., Nakashima, A., Ueno, T., Yokoyama, Y., Kohno, N., Masaki, T., Masaki, T. Mizoribine Ameliorates Renal Injury and Hypertension along with the Attenuation of Renal Caspase-1 Expression in Aldosterone-Salt-Treated Rats. PLoS One 2014; 9(–):U875-U881
- Cheema, M.U., Damkier, H.H., Nielsen, J., Poulsen, E.T., Enghild, J.J., Fenton, R.A., Praetorius, J., Praetorius, J. Distal Renal Tubules Are Deficient in Aggresome Formation and Autophagy upon Aldosterone Administration. PLoS One 2014; 9(–):U303-U315.
- Kreusser, M.M., Lehmann, L.H., Riffel, J.H., Haass, M., Maser-Gluth, C., Backs, J., Katus, H.A., Buss, S.J., Kreusser, M.M. Aldosterone augments Na(+)-induced reduction of cardiac norepinephrine reuptake. American Journal of Physiology-Heart and Circulatory Physiology 2014; 307(–):H1169-H1177.
- Zeniya, M., Sohara, E., Kita, S., Iwamoto, T., Susa, K., Mori, T., Oi, K., Chiga, M., Takahashi, D., Yang, S.S., Lin, S.H., Rai, T., Sasaki, S., Uchida, S. Dietary Salt Intake Regulates WNK3-SPAK-NKCC1 Phosphorylation Cascade in Mouse Aorta Through Angiotensin II. Hypertension 2013; 62(-5-):872-878.
- Hattori, T., Murase, T., Sugiura, Y., Nagasawa, K., Takahashi, K., Ohtake, M., Ohtake, M., Miyachi, M., Murohara, T., Nagata, K. Effects of salt status and blockade of mineralocorticoid receptors on aldosterone-induced cardiac injury. HYPERTENSION RESEARCH 2014; 37(-2-):125-133.
- Salyer, S.A., Parks, J., Barati, M.T., Lederer, E.D., Clark, B.J., Klein, J.D., Khundmiri, S.J. Aldosterone regulates Na+, K+ ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR CELL RESEARCH 2013; 1833(-10-):2143-2152.
- Xue, B.J., Zhang, Z.M., Beltz, T.G., Johnson, R.F., Guo, F., Hay, M., Johnson, A.K. Estrogen Receptor-beta in the Paraventricular Nucleus and Rostroventrolateral Medulla Plays an Essential Protective Role in Aldosterone/Salt-Induced Hypertension in Female Rats. Hypertension 2013; 61(-6-):1255-U261.
- Jin, H.M., Zhou, D.C., Gu, H.F., Qiao, Q.Y., Fu, S.K., Liu, X.L., Pan, Y. Antioxidant N-Acetylcysteine Protects Pancreatic beta-Cells Against Aldosterone-Induced Oxidative Stress and Apoptosis in Female db/db Mice and Insulin-Producing MIN6 Cells. Endocrinology 2013; 154(-11-):4068-4077. >>> Aldosterone; SC; Mice; 2ML4; Animal info (C57BLKS/J, db/db, female, 8 wks old); long-term study; diabetes
- Nitta, E., Hirooka, K., Tenkumo, K., Fujita, T., Nishiyama, A., Nakamura, T., Itano, T., Shiraga, F. Aldosterone: a mediator of retinal ganglion cell death and the potential role in the pathogenesis in normal-tension glaucoma. Cell Death&Disease 2013; 4(-;-):U109-U114.
- Xue, B.J., Zhang, Z.M., Roncari, C.F., Guo, F., Johnson, A.K. Aldosterone Acting Through the Central Nervous System Sensitizes Angiotensin II-Induced Hypertension. Hypertension 2012; 60(-4-):1023-U482.
- van der Lubbe, N., Jansen, P.M., Salih, M., Fenton, R.A., van den Meiracker, A.H., Danser, A.H.J., Zietse, R., Hoorn, E.J. The Phosphorylated Sodium Chloride Cotransporter in Urinary Exosomes Is Superior to Prostasin as a Marker for Aldosteronism. Hypertension 2012; 60(-3-):741-U315.
- Ko, B., Mistry, A.C., Hanson, L., Mallick, R., Wynne, B.M., Thai, T.L., Bailey, J.L., Klein, J.D., Hoover, R.S. Aldosterone acutely stimulates NCC activity via a SPAK-mediated pathway. American Journal of Physiology-Renal Physiology 2013; 305(-5-):F645-F652.
- Kusunoki, H., Taniyama, Y., Rakugi, H., Morishita, R. Cardiac and Renal Protective Effects of Irbesartan via Peroxisome Proliferator-Activated Receptorgamma-Hepatocyte Growth Factor Pathway Independent of Angiotensin II Type 1a Receptor Blockade in Mouse Model of Salt-Sensitive Hypertension. JOURNAL OF THE AMERICAN HEART ASSOCIATION 2013; 2(-2-):U262-U273.
- McGraw, A.P., Bagley, J., Chen, W.S., Galayda, C., Nickerson, H., Armani, A., Caprio, M., Carmeliet, P., Jaffe, I.Z. Aldosterone Increases Early Atherosclerosis and Promotes Plaque Inflammation Through a Placental Growth Factor-Dependent Mechanism. JOURNAL OF THE AMERICAN HEART ASSOCIATION 2013; 2(-1-):U658-U670.
- Nishioka, T., Suzuki, M., Onishi, K., Takakura, N., inada, h, Yoshida, T., Hiroe, M., imanaka-yishida, k J Cardiovasc. Pharmacol 2007; 49(-;-):261-268.
- Bay-Richter, C., Hallberg, L., Ventorp, F., Janelidze, S., Brundin, L. Aldosterone synergizes with peripheral inflammation to induce brain IL-1beta expression and depressive-like effects. Cytokine 2012; 60(-3-):749-754.
- Messaoudi, S., Gravez, B., Tarjus, A., Pelloux, V., Ouvrard-Pascaud, A., Delcayre, C., Samuel, J., Launay, J.M., Sierra-Ramos, C., de la Rosa, D.A., Clement, K., Farman, N., Jaisser, F. Aldosterone-Specific Activation of Cardiomyocyte Mineralocorticoid Receptor In Vivo. Hypertension 2013; 61(-2-):361-U305.
- Queisser, N., Amann, K., Hey, V., Habib, S.L., Schupp, N. Blood pressure has only minor influence on aldosterone-induced oxidative stress and DNA damage in vivo. Free Radical Biology and Medicine 2013; 54(-;-):17-25.
- Calvier, L., Miana, M., Reboul, P., Cachofeiro, V., Martinez-Martinez, E., de Boer, R.A., Poirier, F., Lacolley, P., Zannad, F., Rossignol, P., Lopez-Andres, N. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. ARTERIOSCLEROSIS THROMBOSIS AND VASCULAR BIOLOGY 2013; 33(-1-):67-U221.
- Formenti, S., Bassi, M., Nakamura, N.B., Schoorlemmer, G.H.M., Menani, J.V., Colombari, E. Hindbrain mineralocorticoid mechanisms on sodium appetite. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 2013; 304(-3-):R252-R259.
- Patel, A.B., Frindt, G., Palmer, L.G. Feedback inhibition of ENaC during acute sodium loading in vivo. American Journal of Physiology-Renal Physiology 2013; 304(-2-):F222-F232. >>> Aldosterone; amiloride; PEG 300; SC; Rat; 2002; Animal info (Sprague Dawley, female, wks old, 200-300 g).
- Ronzaud, C., Loffing-Cueni, D., Hausel, P., Debonneville, A., Malsure, S.R., Fowler-Jaeger, N., Boase, N.A., Perrier, R., Maillard, M., Yang, B.L., Stokes, J.B., Koesters, R., Kumar, S., Hummler, E., Loffing, J., Staub, O. Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. Journal of Clinical Investigation 2013; 123(-2-):657-665.
- McCurley, A., Pires, P.W., Bender, S.B., Aronovitz, M., Zhao, M.J., Metzger, D., Chambon, P., Hill, M.A., Dorrance, A.M., Mendelsohn, M.E., Jaffe, I.Z. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nature Medicine 2012; 18(-9-):1429-U182.
- Yuan, Y.G., Huang, S.M., Wang, W.Y., Wang, Y.Y., Zhang, P., Zhu, C.H., Ding, G.X., Liu, B.C., Yang, T.X., Zhang, A.H. Activation of peroxisome proliferator-activated receptor-gamma coactivator 1alpha ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney International 2012; 82(-7-):771-789.
- Veitenheimer, B.J., Engeland, W.C., Guzman, P.A., Fink, G.D., Osborn, J.W. Effect of global and regional sympathetic blockade on arterial pressure during water deprivation in conscious rats. American Journal of Physiology-Heart and Circulatory Physiology 2012; 303(-8-):H1022-H1034.
- Uchimura, K., Kakizoe, Y., Onoue, T., Hayata, M., Morinaga, J., Yamazoe, R., Ueda, M., Mizumoto, T., Adachi, M., Miyoshi, T., Shiraishi, N., Sakai, Y., Tomita, K., Kitamura, K. In vivo contribution of serine proteases to the proteolytic activation of gammaENaC in aldosterone-infused rats. American Journal of Physiology-Renal Physiology 2012; 303(-7-):F939-F943.
- Garcia, A.G., Wilson, R.M., Heo, J., Murthy, N.R., Baid, S., Ouchi, N., Sam, F. Interferon-gamma ablation exacerbates myocardial hypertrophy in diastolic heart failure. American Journal of Physiology-Heart and Circulatory Physiology 2012; 303(-5-):H587-H596.
- Huang, B.S., Zheng, H., Tan, J.H., Patel, K.P., Leenen, F.H.H. Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Experimental Physiology 2011; 96(-10-):1028-1038.
- Deshmukh, P.A., Bellary, S.R., Schwender, F.T., Kamalov, G., Magotra, M., Curry, A.L.D., Sun, Y., Weber, K.T. Spironolactone Prevents the Inducibility of Ventricular Tachyarrhythmia in Rats With Aldosteronism. Journal of Cardiovascular Pharmacology 2011; 58(-5-):487-491.
- Ogawa, Y., Mukoyama, M., Yokoi, H., Kasahara, M., Mori, K., Kato, Y., Kuwabara, T., Imamaki, H., Kawanishi, T., Koga, K., Ishii, A., Tokudome, T., Kishimoto, I., Sugawara, A., Nakao, K. Natriuretic Peptide Receptor Guanylyl Cyclase-A Protects Podocytes from Aldosterone-Induced Glomerular Injury. Journal of the American Society of Nephrology 2012; 23(-7-):1198-1209.
- Andres, N.L., Tesse, A., Regnault, V., Louis, H., Cattan, V., Thornton, S.N., Labat, C., Kakou, A., Tual-Chalot, S., Faure, S., Challande, P., Osborne-Pellegrin, M., Martinez, M.C., Lacolley, P., Andriantsitohaina, R. Increased Microparticle Production and Impaired Microvascular Endothelial Function in Aldosterone-Salt-Treated Rats: Protective Effects of Polyphenols. PLoS One 2012; 7(-7-):U69-U82.
- Waeckel, L., Potier, L., Chollet, C., Taveau, C., Bruneval, P., Roussel, R., henc-Gelas, F., Bouby, N. Antihypertensive Role of Tissue Kallikrein in Hyperaldosteronism in the Mouse. Endocrinology 2012; 153(-8-):3886-3896. >>> Aldosterone; Saline, isotonic; SC; Mice; 2004; Controls received mp w/ vehicle; animal info (TK -/-, wt).
- 12354 Lammers, C., Dartsch, T., Brandt, M.C., Rottlaender, D., Halbach, M., Peinkofer, G., Ockenpoehler, S., Weiergraeber, M., Schneider, T., Reuter, H., Mueller-Ehmsen, J., Hescheler, J., Hoppe, U.C., Zobel, C. Spironolactone Prevents Aldosterone Induced Increased Duration of Atrial Fibrillation in Rat. CELLULAR PHYSIOLOGY AND BIOCHEMISTRY 2012; 29(-5-6-):833-840.
- van der Lubbe, N., Lim, C.H., Meima, M.E., van Veghel, R., Rosenbaek, L.L., Mutig, K., Danser, A.H.J., Fenton, R.A., Zietse, R., Hoorn, E.J. Aldosterone does not require angiotensin II to activate NCC through a WNK4-SPAK-dependent pathway. PFLUGERS ARCHIV-EUROPEAN JOURNAL OF PHYSIOLOGY 2012; 463(-6-):853-863.
- Ding, W., Yang, L., Zhang, M.M., Gu, Y. Chronic inhibition of nuclear factor kappa B attenuates aldosterone/salt-induced renal injury. LIFE SCIENCES 2012; 90(-15-16-):600-606. >>> Aldosterone; Ethanol; SC; Rat; 2004; Controls received mp w/ vehicle; animal info (Sprague Dawley, male, 5 wks old, 180-208 g); 0.5% ethanol used; replacement therapy (nephrectomy).
- 12243 Sekizawa, N., Yoshimoto, T., Hayakawa, E., Suzuki, N., Sugiyama, T., Hirata, Y. Transcriptome analysis of aldosterone-regulated genes in human vascular endothelial cell lines stably expressing mineralocorticoid receptor. MOLECULAR AND CELLULAR ENDOCRINOLOGY 2011; 341(-1-2-):78-88.
- Kasal, D.A., Barhoumi, T., Li, M.W., Yamamoto, N., Zdanovich, E., Rehman, A., Neves, M.F., Laurant, P., Paradis, P., Schiffrin, E.L. T Regulatory Lymphocytes Prevent Aldosterone-Induced Vascular Injury. Hypertension 2012; 59(-2-):324-U414.
- Liu, Y., Hirooka, K., Nishiyama, A., Lei, B., Nakamura, T., Itano, T., Fujita, T., Zhang, J.S., Shiraga, F. Activation of the aldosterone/mineralocorticoid receptor system and protective effects of mineralocorticoid receptor antagonism in retinal ischemia-reperfusion injury. Experimental Eye Research 2012; 96(-1-):116-123.
- Terada, Y., Ueda, S., Hamada, K., Shimamura, Y., Ogata, K., Inoue, K., Taniguchi, Y., Kagawa, T., Horino, T., Takao, T. Aldosterone stimulates nuclear factor-kappa B activity and transcription of intercellular adhesion molecule-1 and connective tissue growth factor in rat mesangial cells via serum- and glucocorticoid-inducible protein kinase-1. Clinical and Experimental Nephrology 2012; 16(-1-):81-88.
- Ladage, D., Schuetzeberg, N., Dartsch, T., Krausgrill, B., Halbach, M., Zobel, C., Mueller-Ehmsen, J. Hyperaldosteronism is associated with a decrease in number and altered growth factor expression of endothelial progenitor cells in rats. International Journal of Cardiology 2011; 149(-2-):152-156.
- Kawarazaki, H., Ando, K., Fujita, M., Matsui, H., Nagae, A., Muraoka, K., Kawarasaki, C., Fujita, T. Mineralocorticoid receptor activation: a major contributor to salt-induced renal injury and hypertension in young rats. American Journal of Physiology-Renal Physiology 2011; 300(-6-):F1402-F1409.
- Yan, Y.J., Ouyang, J., Wang, C., Wu, Z., Ma, X., Li, H.Z., Xu, H., Hu, Z., Li, J., Wang, B.J., Shi, T.P., Gong, D.J., Ni, D., Zhang, X. Aortic Cell Apoptosis in Rat Primary Aldosteronism Model. Journal of Huazhong University of Science and Technology-Medical Sciences 2010; 30(-3-):385-390.
- Pech, V., Pham, T.D., Hong, S., Weinstein, A.M., Spencer, K.B., Duke, B.J., Walp, E., Kim, Y.H., Sutliff, R.L., Bao, H.F., Eaton, D.C., Wall, S.M. Pendrin Modulates ENaC Function by Changing Luminal HCO(3)(-). Journal of the American Society of Nephrology 2010; 21(-11-):1928-1941.
- van der Lubbe, N., Lim, C.H., Fenton, R.A., Meima, M.E., Danser, A.H.J., Zietse, R., Hoorn, E.J. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney International 2011; 79(-1-):66-76.
- Thum, T., Schmitter, K., Fleissner, F., Wiebking, V., Dietrich, B., Widder, J.D., Jazbutyte, V., Hahner, S., Ertl, G., Bauersachs, J. Impairment of endothelial progenitor cell function and vascularization capacity by aldosterone in mice and humans. European Heart Journal 2011; 32(-10-):1275-1286.
- Shibata, S., Mu, S.Y., Kawarazaki, H., Muraoka, K., Ishizawa, K., Yoshida, S., Kawarazaki, W., Takeuchi, M., Ayuzawa, N., Miyoshi, J., Takai, Y., Ishikawa, A., Shimosawa, T., Ando, K., Nagase, M., Fujita, T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. Journal of Clinical Investigation 2011; 121(-8-):3233-3243.
- He, B.J., Joiner, M.L.A., Singh, M.V., Luczak, E.D., Swaminathan, P.D., Koval, O.M., Kutschke, W., Allamargot, C., Yang, J.Y., Guan, X.Q., Zimmerman, K., Grumbach, I.M., Weiss, R.M., Spitz, D.R., Sigmund, C.D., Blankesteijn, W.M., Heymans, S., Mohler, P.J., Anderson, M.E. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nature Medicine 2011; 17(-12-):1610-U125.
- Brem, A.S., Morris, D.J., Ge, Y., Dworkin, L., Tolbert, E., Gong, R.J. Direct fibrogenic effects of aldosterone on normotensive kidney: an effect modified by 11 beta-HSD activity. American Journal of Physiology-Renal Physiology 2010; 298(-5-):F1178-F1187.
- Griol-Charhbili, V., Fassot, C., Messaoudi, S., Perret, C., Agrapart, V., Jaisser, F. Epidermal Growth Factor Receptor Mediates the Vascular Dysfunction But Not the Remodeling Induced by Aldosterone/Salt. Hypertension 2011; 57(-2-):238-U205.
- Fukuda, S., Horimai, C., Harada, K., Wakamatsu, T., Fukasawa, H., Muto, S., Itai, A., Hayashi, M. Aldosterone-induced kidney injury is mediated by NFkappaB activation. Clinical and Experimental Nephrology 2011; 15(-1-):41-49.
- Huang, B.S., Ahmadi, S., Ahmad, M., White, R.A., Leenen, F.H.H. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-“ouabain”pathway. American Journal of Physiology-Heart and Circulatory Physiology 2010; 299(-2-):U207-U215.
- Wang, K., Long, B., Zhou, J., Li, P.F. miR-9 and NFATc3 Regulate Myocardin in Cardiac Hypertrophy. Journal of Biological Chemistry 2010; 285(-16-):11903-11912.
- Sam, F., Duhaney, T.A.S., Sato, K., Wilson, R.M., Ohashi, K., Sono-Romanelli, S., Higuchi, A., De Silva, D.S., Qin, F.Z., Walsh, K., Ouchi, N. Adiponectin Deficiency, Diastolic Dysfunction, and Diastolic Heart Failure. Endocrinology 2010; 151(-1-):322-331.
- Matsubara, B.B., Franco, M., Janicki, J.S., Matsubara, L.S. Effect of felodipine on myocardial and renal injury induced by aldosterone-high salt hypertension in uninephrectomized rats. Brazilian Journal of Medical and Biological Research 2010; 43(-5-):506-514.
- Hunter, R.G., Bloss, E.B., McCarthy, K.J., McEwen, B.S. Regulation of the nicotinic receptor alpha7 subunit by chronic stress and corticosteroids. Brain Research 2010; 1325(-;-):141-146.
- Kamalov, G., Ahokas, R.A., Zhao, W.Y., Shahbaz, A.U., Bhattacharya, S.K., Sun, Y., Gerling, I.C., Weber, K.T. Temporal responses to intrinsically coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria during aldosteronism. American Journal of Physiology-Heart and Circulatory Physiology 2010; 298(-2-):H385-H394.
- Guerrero P, Fuchs FD, Moreira LM, Martins VM, Bertoluci C, Fuchs SC, Gus M. Blood pressure-lowering efficacy of amiloride versus enalapril as add-on drugs in patients with uncontrolled blood pressure receiving hydrochlorothiazide. Clin Exp Hypertens. 2008 Oct;30(7):553-64.
- Kaşifoğlu T, Yalçin AU. The effects of thiazide and thiazide-potassium sparing diuretics on fibrinolytic system parameters. Anadolu Kardiyol Derg. 2006 Jun;6(2):143-7.
- Bergström J. The effect of hydrochlorothiazide and amiloride administered together on muscle electrolytes in normal subjects. acta Med Scand. 1975 May;197(5):415-9.
- The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J [Internet] 2012;33:1787–1847. [cited 2013 Oct 11];
- Heart and Stroke Foundation [Internet]. Ottawa: Heart and Stroke Foundation of Canada. Statistics; 2012.
- Tracking heart disease and stroke in Canada 2009 [Internet]. Ottawa: Public Health Agency of Canada; Nov 24, 2012. [cited 2013 Oct 18].
- McKelvie RS, Moe GW, Ezekowitz JA, Heckman GA, Costigan J, Ducharme A, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol. 2013 Feb;29(2):168–181.
- CDR submission binder: Inspra (eplerenone) tablets, 25 mg and 50 mg; new indication. Company: Pfizer Canada Inc. [CONFIDENTIAL manufacturer’s submission]. Kirkland (QC): Pfizer Canada Inc; Jul, 2013.
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011 Jan 6;364(1):11–21.
- Clinical study report SC-066110: the effect of eplerenone versus placebo on cardiovascular mortality and heart failure hospitalization in subjects with NYHA class II chronic systolic heart failure (EMPHASIS-HF) [CONFIDENTIAL internal manufacturer’s report]. New York: Pfizer; 2011.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Dranzer MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol [Internet] 2013 Oct;62(16):e147–e239. [cited 2013 Oct 11];
- Colucci WS. Predictors of survival in heart failure due to systolic dysfunction. 2013 Jul 11 [cited 2013 Oct 29] Waltham (MA): UpToDate [Internet]. Release:21.8 – C21.149. UpToDate; 1992 -.
- Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol. 2008 Apr;31(4):153–158.
- Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, et al. Safety and efficacy of eplerenone in patients at high-risk for hyperkalemia and/or worsening renal function: analyses of EMPHASIS-HF study subgroups. J Am Coll Cardiol. 2013;62(17):1585–1593.
- Krum H, Shi H, Pitt B, McMurray J, Swedberg K, van Veldhuisen DJ, et al. Clinical benefit of eplerenone in patients with mild symptoms of systolic heart failure already receiving optimal best practice background drug therapy: analysis of the EMPHASIS-HF study. Circ Heart Fail. 2013 Jul 1;6(4):711–718.
- Preiss D, van Veldhuisen DJ, Sattar N, Krum H, Swedberg K, Shi H, et al. Eplerenone and new-onset diabetes in patients with mild heart failure: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Eur J Heart Fail. 2012 Aug;14(8):909–915.
- Rogers JK, McMurray JJ, Pocock SJ, Zannad F, Krum H, van Veldhuisen DJ, et al. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation. 2012 Nov 6;126(19):2317–2323.
- Swedberg K, Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Shi H, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012 May 1;59(18):1598–1603.
- Zannad F, McMurray JJ, Drexler H, Krum H, van Veldhuisen DJ, Swedberg K, et al. Rationale and design of the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF). Eur J Heart Fail [Internet] 2010 Jun;12(6):617–622. [cited 2013 Sep 5];
- Dhillon S. Eplerenone: a review of its use in patients with chronic systolic heart failure and mild symptoms. Drugs. 2013;73(13):1451–1462.
- Sullivan EJ. Clinical trial endpoints [slides on the Internet]. Rockville (MD): U.S. Food and Drug Administration; 2013. [cited 2013 Oct 11].
- Manufacturer’s comments on CDR clinical and pharmacoeconomic reviewers’ reports on eplerenone (Inspra) [CONFIDENTIAL manufacturer’s report]. Kirkland (QC): Pfizer Canada; Nov 15, 2013.
- Inspra (eplerenone): tablets 25 mg and 50 mg [product monograph]. Kirkland (QC): Pfizer Canada Inc; Aug 21, 2012.
- Nagarajan V, Chamsi-Pasha M, Tang WH. The role of aldosterone receptor antagonists in the management of heart failure: an update. Cleve Clin J Med [Internet] 2012 Sep;79(9):631–639. [cited 2013 Sep 5];
- Gupta S, Fugh-Berman AJ, Scialli A. Ethics and eplerenone. J Med Ethics. 2013 Feb;39(2):110–114.
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med [Internet] 1999 Sep 2;341(10):709–717. [cited 2013 Oct 21];
- Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, et al. Effect of eplerenone versus spironolactone on cortisol and hemoglobin A1(c) levels in patients with chronic heart failure. Am Heart J. 2010 Nov;160(5):915–921.
- Baliga RR, Ranganna P, Pitt B, Koelling TM. Spironolactone treatment and clinical outcomes in patients with systolic dysfunction and mild heart failure symptoms: a retrospective analysis. J Card Fail. 2006 May;12(4):250–256.
- Chatterjee S, Moeller C, Shah N, Bolorunduro O, Lichstein E, Moskovits N, et al. Eplerenone is not superior to older and less expensive aldosterone antagonists. Am J Med. 2012 Aug;125(8):817–825.
- Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J [Internet] 2009 Feb;30(4):469–677. [cited 2013 Sep 6];
- Hu LJ, Chen YQ, Deng SB, Du JL, She Q. Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013 May;75(5):1202–1212.
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309–1321.
- CDR submission binder: Inspra (eplerenone) tablets, 25 mg and 50 mg new indication. Company: Pfizer Canada Inc. [CONFIDENTIAL manufacturer’s submission]. Kirkland (QC): Pfizer Canada Inc; Jul, 2013. Pharmacoeconomic evaluation.
- Common Drug Review Eplerenone (Inspra – Pfizer Canada Inc.). Indication: heart failure post myocardial infarction. CEDAC final recommendation [Internet]. Ottawa: Canadian Agency for Drugs and Technologies in Health; Nov, 2009. [cited 2013 Oct 22].
- Pfizer response to October 11, 2013 request for additional information regarding the Inspra HF CDR review. Synopsis clinical study report: eplerenone. [CONFIDENTIAL additional manufacturer’s information]. Pointe-Claire/Dorval (QC): Pfizer Canada Inc; Oct 23, 2013.
- Aldactone Consumer Medicine Information (CMI). West Ryde, NSW: Pfizer Australia Pty Ltd. November 2012. [PDF]
- Aldactone Product Information (PI). West Ryde, NSW: Pfizer Australia Pty Ltd. October 2014. [PDF]
- Aronoff A, Nayarai I. Le traitement de l’ascite resistante des cirrhotiques. L’Union Medicale du
Canada 1974;103:2081-9. - Berg KJ, Gisholt K, Wideroe TE. Potassium deficiency in hypertensives treated with diuretics.
Analysis of three alternative treatments by an oral test for potassium deficiency. Eur J Clin
Pharmacol 1974;7:401-5. - Bravo EL, Dustan HP, Tarazi RC. Spironolactone as a non-specific treatment for primary
aldosteronism. Circulation 1973;48:491-8. - Burden RP, Booth LJ, Aber GM. The diagnostic and therapeutic value of spironolactone in
patients with systemic hypertension. Nephron 1972;9:171-88. - Cicoira M, Zanolla L, Rossi A et al.: Long-term, dose-dependent effects of spironolactone on
left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll
Cardiol 2002; 40(2):304-10. - Cominos-Torres R, Ma L, Snyder PJ. Gynecomastia and semen abnormalities induced by
spironolactone in normal men. J Clin Endocrinol Metab 1977;45:255-60. - Conn JW, Hinerman DL, Cohen EL. The effects of spironolactone upon adrenal function and
morphology. Systemic effects of antihypertensive agents. (ed. Sambhi MP) Symposia
Specialists 1976;359-82. - Douglas JG, Hollifield JW, Liddle GW. Treatment of low renin essential hypertension.
Comparison of spironolactone and hydrochlothiazide-triamterene combination. J Am Med
Assoc 1974;227:518-21. - Kenneth L. Becker, Principles and practice of endocrinology and metabolism, Lippincott Williams & Wilkins, 2001, pp. 785–, ISBN 978-0-7817-1750-2.
- DeFronzo RA, Hyperkalemia and hyporeninemic hypoaldosteronism, in Kidney Int., vol. 17, nº 1, 1980, pp. 118–34, DOI:10.1038/ki.1980.14,
- Sebastian A, Schambelan M, Sutton JM, Amelioration of hyperchloremic acidosis with furosemide therapy in patients with chronic renal insufficiency and type 4 renal tubular acidosis, in Am. J. Nephrol., vol. 4, nº 5, 1984, pp. 287–300,